

Specifically, studies investigating narcolepsy with positron emission tomography (PET) and single photon emission computed tomography (SPECT) have observed aberrant perfusion and glucose metabolism in the hypothalamus and thalamus, as well as in prefrontal cortex (PFC Joo et al., 2004, 2005 Hong et al., 2006 Dauvilliers et al., 2010). Results from previous functional and structural neuroimaging studies would suggest that the loss of hypocretin-1 has numerous downstream effects in terms of both resting state glucose metabolism and perfusion and reduction in cortical gray matter. In addition to the sleep-related changes summarized here, the loss of hypocretin-1 is also thought to be an underlying cause to the changes in cognition observed in patients with narcolepsy ( Fulda and Schulz, 2001 Rieger et al., 2003 Naumann et al., 2006 Bayard et al., 2012).

Most narcolepsy patients also suffer from cataplexy, a sudden reduction or loss of muscular tone not accompanied by loss of consciousness caused by the loss of hypocretin-1 (orexin) producing neurons in the lateral hypothalamus ( Lin et al., 1999 Thannickal et al., 2000 Nishino et al., 2010). Other symptoms include rapid eye movement (REM) sleep abnormalities such as sleep paralysis, hypnagogic (upon falling asleep) or hypnopompic (upon awakening) hallucinations, and nocturnal dyssomnia with fragmented sleep and frequent awakenings. Narcolepsy is a chronic sleep disorder, characterized by excessive daytime sleepiness with frequent uncontrollable sleep attacks ( Silber et al., 2002 Dauvilliers et al., 2007). We concluded that adolescents with narcolepsy have altered resting state brain dynamics.
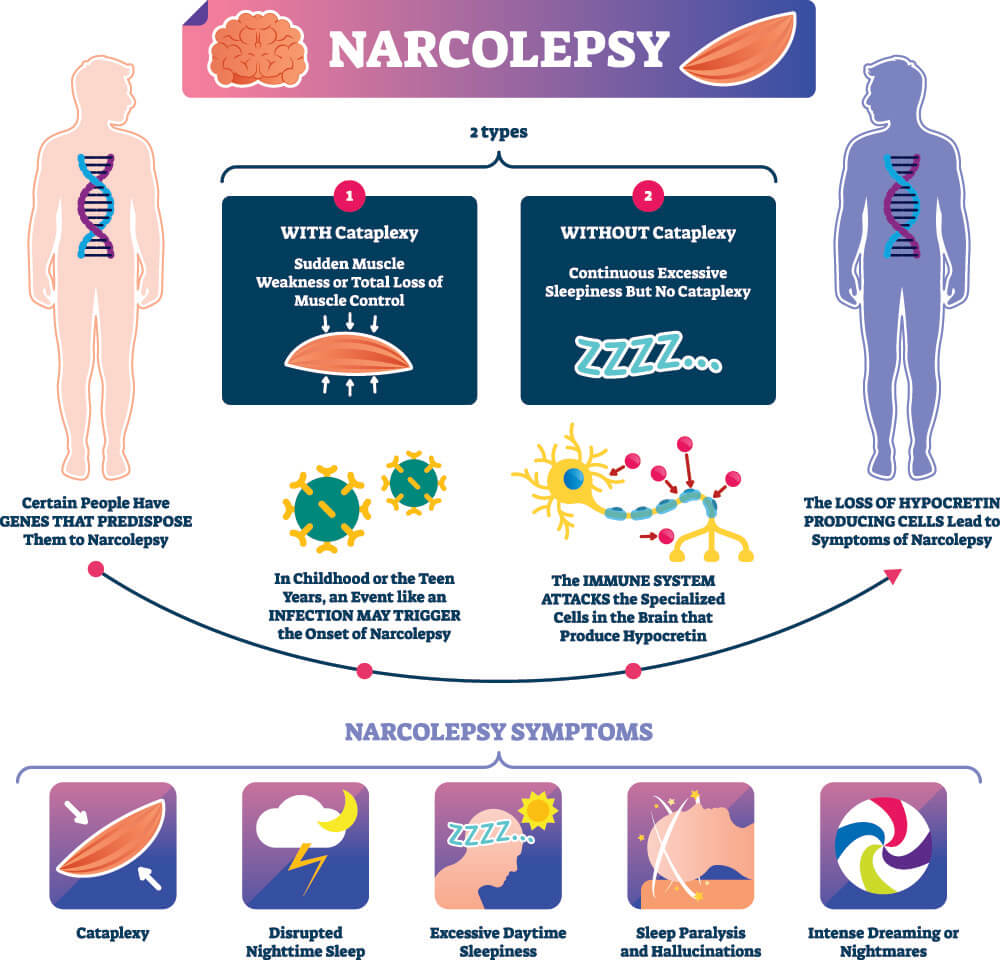
Data showed that narcolepsy patients were less likely than controls to spend time in a microstate which we found to be related to the default mode network and may suggest a disruption of this network that is disease specific. Functional MRI data were analyzed for RSNs. EEG data were analyzed for microstates, which are discrete epochs of stable global brain states obtained from topographical EEG analysis. Simultaneous EEG and fMRI data were collected during 10 min of wakeful rest. Sixteen adolescents (ages 13–20) with a confirmed diagnosis of narcolepsy were recruited and compared to age-matched healthy controls. The objective of this study was to investigate and describe brain microstate activity in adolescents with narcolepsy and correlate these to RSNs using simultaneous fMRI and electroencephalography (EEG). Given these findings in brain structure and metabolism in narcolepsy, we anticipated that changes in functional magnetic resonance imaging (fMRI) resting state network (RSN) dynamics might also be apparent in patients with narcolepsy. In addition to hypothalamic and thalamic dysfunction they showed aberrant prefrontal perfusion and glucose metabolism in narcolepsy. Previous neuroimaging studies have investigated brain function in narcolepsy during rest using positron emission tomography (PET) and single photon emission computed tomography (SPECT). Narcolepsy is a chronic sleep disorder caused by a loss of hypocretin-1 producing neurons in the hypothalamus.


 0 kommentar(er)
0 kommentar(er)
